This is a summary of an article published in Chemical Engineering Science (2022).
1. Introduction
The scaling of heat exchangers is a persistent and often expensive problem which effects the efficiency and rollout of sustainable heating systems. Chemical cleaning processes exist but are costly, polluting, and require plant shutdown, resulting in operational losses. With sustainable development in mind, there is ongoing exploration of alternatives that are inexpensive, robust, and ecological. Many are now turning to physical water treatment to simultaneously reduce energy costs, lessen environmental impact, and ensure the efficient operation of their infrastructure.
1.1 Physical water treatment
Instead of treating water via chemical means, physical processes act to prevent scale and biofilm deposits without modifying the minerality of the water.
A study by Nihad Kamar and colleagues at University of Lorraine (France) paired an AQUA4D® system with a gasketed plate heat exchanger, to study how this treated water influences the incidence of problematic calcium carbonate. To date, numerous studies have been conducted on the effects of this physical treatment on biofilm formation and mineral deposit formation (check our Scientific Studies section here)
Now in 2022, Kamar et al. aimed to compare the effects of untreated vs (AQUA4D®) treated water on the incidence of calcium carbonate (CaCO3) inside a plate and joint heat exchanger.
2. Material and methods
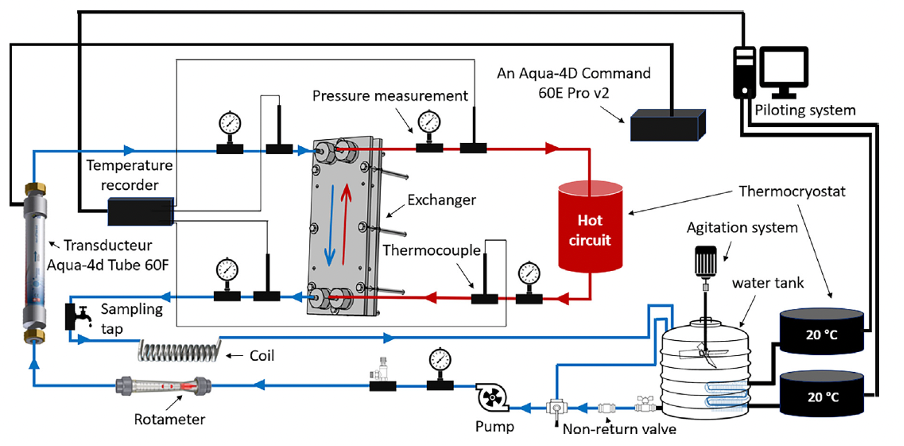
The research looked at the formation of CaCO3 under different water temperatures within a plate heat exchanger. For a test duration of 72 hours and a flow rate of 100 L.h-1, this research used an AQUA4D® 60E Pro mounted directly at the inlet of the cold circuit of the exchanger to diffuse very low frequency resonance fields into the water.
Aspects including temperature, conductivity, pH, and ion concentrations (Ca2+, Mg2+, HCO3–) were monitored, which allows understanding of mechanisms involved in the precipitation of CaCO3. Importantly, calcium carbonate salt has an inverse solubility: the more it’s heated, the more it precipitates. To determine the amount of solid deposited within the exchanger, a cleaning/dissolution protocol was carried out, involving the draining of the cold circuit with compressed gas, determination the concentration of dissolved Ca2+ and Mg2+ in the solution by ICP-OES spectroscopy, and calculation of the amount deposited inside the exchanger in gram/per day/per unit area. The diagram below illustrates the procedure:
3. Results and discussion
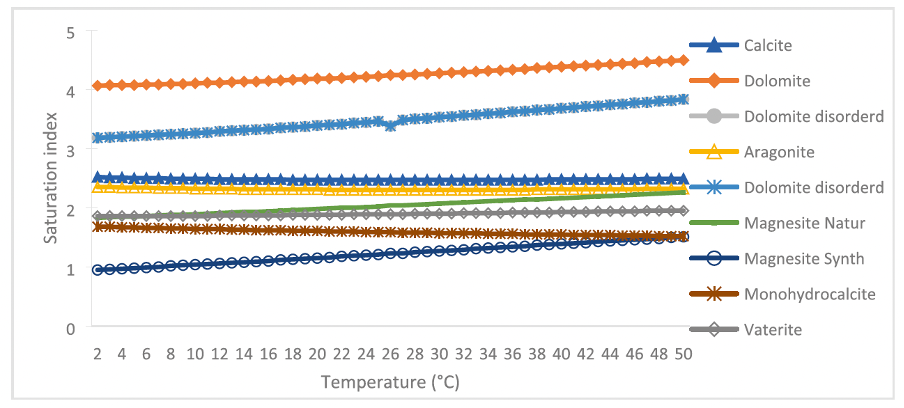
The treated and untreated water was analyzed by Phreeqc software, showing that the same nine elements are hypothetically possible to precipitate in the temperature range distinguished for this study.
3.2 Result: Reduced deposit quantities
Below are summarized the results of four experiments with identical temperature conditions (hot circuit 50°C, cold circuit 20°C), flow rate 100 L.h-1, test duration 72 hours). The total deposits in the heat exchanger are estimated by lixiviation; a mass balance allowed researchers to estimate the total quantity of calcium carbonate. The table below summarizes the material balance:
After three days, calcium is present in dissolved form as well as in precipitated form. After AQUA4D® treatment, a reduction in the amount of scale deposited on the exchanger plates from 4.2 g/day/m2 g to 0.89 g/day/m2 was obtained, which represents a deposit reduction ratio of about 76.83 %.
3.3 Results upon opening the heat exchanger
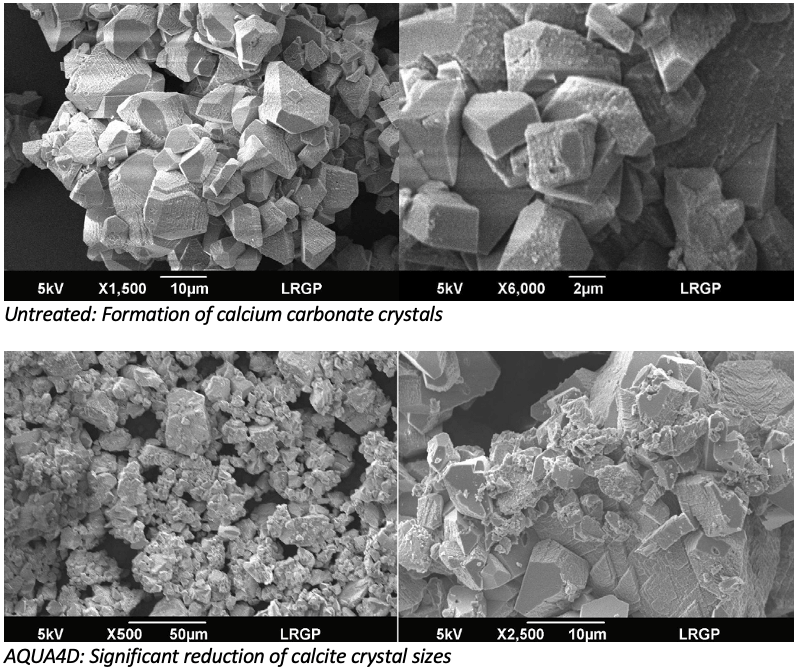
In the presence of AQUA4D®, a significant reduction of deposits is observed, especially in the first cold compartment of the exchanger. At the inlet of the exchanger, a reduction of the calcite crystal size was observed. The particle size analysis indicates that the volume average diameter is about 63.5 ± 4 µm. A 36 % reduction in crystal size was observed.
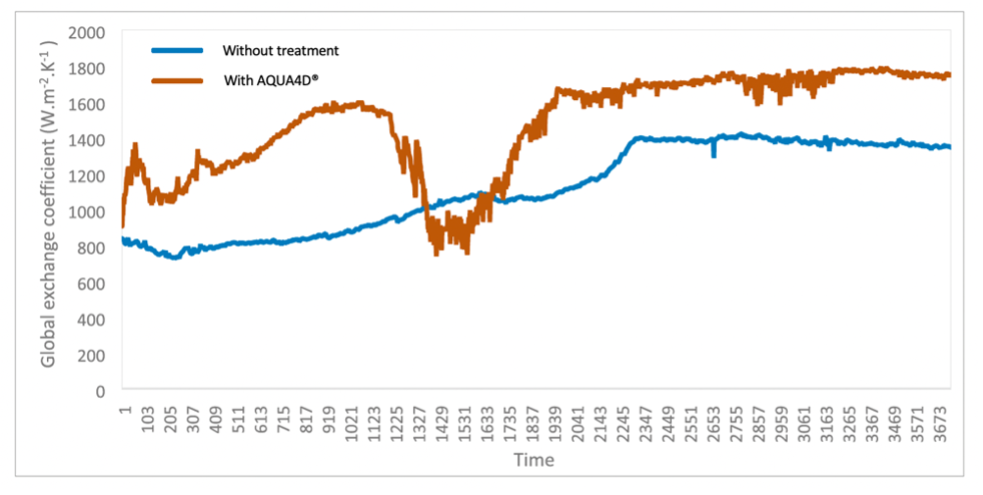
Importantly, analysis of the global exchange coefficient showed that AQUA4D® made heat exchange consistently more effective over time:
5. Conclusion
The results obtained show that AQUA4D® treatment can efficiently reduce precipitate deposits by about 80%. In the present study, the precipitate deposited is calcite, in the form of agglomerates of a smaller average diameter, of the order of 63.5 ± 4 lm. This experimental work shows the feasibility of AQUA4D® to inhibit limescale deposit formation. The results of RAMAN spectroscopy and SEM imaging prove that the physical quality of the deposit is agglomerated and of smaller size with AQUA4D® treatment. To conclude, AQUA4D® treatment inhibits the formation of calcium carbonate deposits in the heat exchanger. This physical water treatment is very efficient and easy to implement, and the physical applications related to mineral phenomena can be numerous.
The wider ramifications of this study imply that through effective water treatment AQUA4D® can solve persistent limescale issues, thereby decreasing the need for chemical treatment and increasing the efficiency and sustainability of installations.
Worldwide
Sustainable Buildings
Scientific Studies

“The results obtained show that AQUA4D® treatment can efficiently reduce precipitate deposits by about 80%. The wider ramifications of this study imply that through effective water treatment AQUA4D® can solve persistent limescale issues, thereby decreasing the need for chemical treatment and increasing the efficiency and sustainability of installations.”